- About Us
- Outreach
- …
- About Us
- Outreach

- About Us
- Outreach
- …
- About Us
- Outreach

Chemistry Activities

What is a chemical reaction? How can you tell one has occurred? Have your students perform a simple chemical reaction using chalk (calcium carbonate) and vinegar. They will also attempt to quantitatively prove the Law of Conservation of Mass. An activity is provided for high school students with a basic understanding of balancing reactions and stoichiometry.
Grade Levels: 6-12

What is the role of an enzyme? Students will learn the principles of how enzymes work by creating their own tests to observe the breakdown of proteins with household enzymes, like hydrogen peroxide.
Grade Levels: 6-12

How does a battery store charge? Where do the charges go? Have your students learn how a battery separates two favorable half reactions to store energy and to learn the variables that affect a batteries performance.
Grade Levels: 9-12

How can we coat one metal onto another? Have your Students make a silvery penny, and a copper nickel. In the process, they will learn about electrochemistry and electroplating.
Grade Levels: 6-12

How does a chemical reaction affect temperature? Students will experiment with salts to look at Exothermic and Endothermic reactions. They will also learn about the difference
between temperature and thermal energy.Grade Levels: 6-8

Can you see your DNA? Students will learn about the importance of this molecule and extract it from an organism. They will then model how the code is used to copy itself and make proteins.
Grade Levels: 7-12

Students learn about techniques of forensic science including: fingerprint identification, chromatography, and chemistry.
Grade Levels: 5-8

What is a fuel cell? Students connect to and build upon their knowledge of oxidation-reduction reactions in order to introduce the chemistry involved in fuel cell technology.
Grade Levels: 9-12

What is DNA fingerprinting? Students will learn how gel electrophoresis works and how it is used in fields like forensics to study DNA of various individuals.They will conduct an
experiment to find out if different food dyes use the same colors.Grade Levels: 7-12

How can we test the mechanical properties of polymers? Students will design and conduct an experiment in order to classify the different forms of matter that they create through the
cross-linking of polymers.Grade Levels: 6-12

What is a hydrogel and what are they used for? Students will make their own hydrogel and test them for its mechanical properties. They will then conduct an experiment to find out whether a variable can change the properties of the hydrogel. Looking atcurrent research on hydrogels as a substitute for cartilage, students will attempt to design a hydrogel that has properties similar to
cartilage.Grade Levels: 7-12

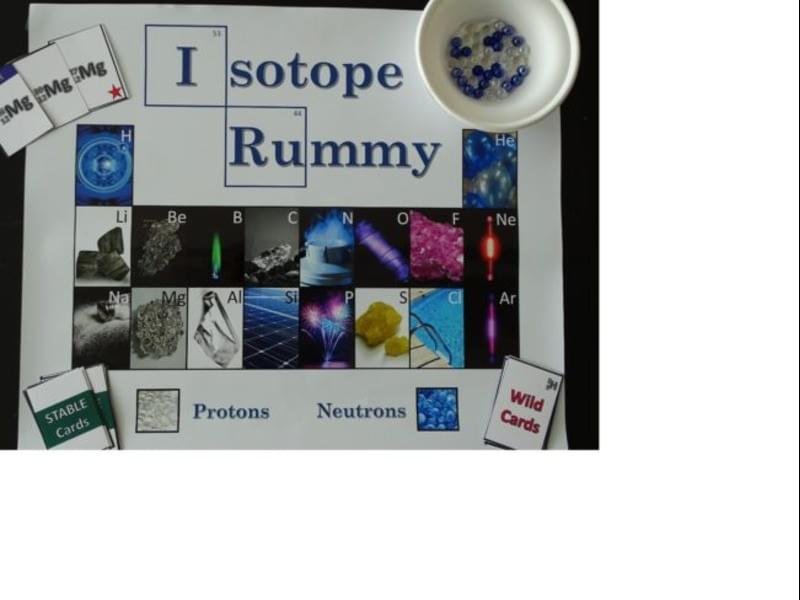
What is an isotope? Students will play an interactive game to learn the answer to this question and also find out how isotopes real-world applications make them useful to scientists.
Grade Levels: 9-12

What is a liquid crystal? Have your students explore these unique molecules by creating and observing hand prints. They will then use liquid crystal thermometers to learn about
the 3 states of matter and find out how temperature affects each state.Upper elementary students can also use the liquid crystal papers to explore how heat is transferred through conduction. They will make a connection with specific changes of state
during the water cycle (evaporation, condensation).Grade Levels: K-2 & 3-5

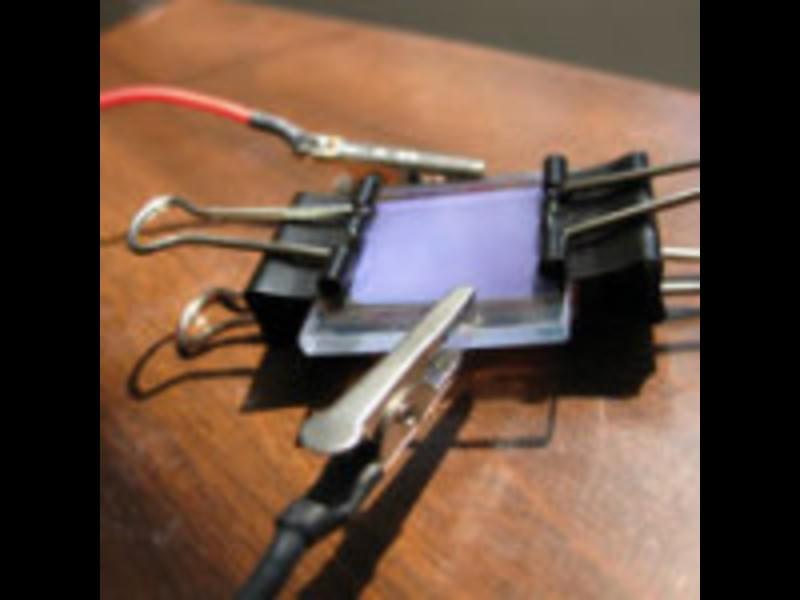
How does a battery work? Students will figure this out as well as see how energy is transformed by building a wet cell battery. They will also test a variable to find out how it affects the strength of a battery.
Grade Levels: 6-12

What is nano? Students will explore the nanoscale world and see how materials look and behave quite differently. Several exploratory activities encourage student to make observations and formulate scientific hypotheses. Middle schoolers will learn about the chemistry of water hardness and how to test for it. They will then explore different ways to remove the hardness in water. High school students will use a gold nanoparticle solution to test different sports drinks for electrolytes.This kit enables students to experience and take advantage of the unique properties of nano-scale materials.
Grade Levels: 6-8 & 9-12

What is a polymer? Introduce your students to polymers and the various roles they play in our everyday lives. The first activity will heve them look at Sodium Polyacyrlate and how it is used to
absorb liquids. Students learn about two forms of this polymer and how achange in their structure can affect their physical properties. Students can then make silly putty and learn how scientists can change polymers to give them different physical properties.Grade Levels: 3-8 & 9-12

Can a blueberry make electricity? Have your students find out by building their own solar cells using berry juice. This activity can be geared towards middle or high school students and meets NGSS standards. Topics that can be covered include photosynthesis, chemical reactions, and
changes in energy. The activity can also be extended by having the students look at how a variable affects the power produced by the solar cell.Grade Levels: 6-12

Can we use light to measure the amount of a known chemical substance (concentrations)? Students will learn about absorption spectrophotometry by building a LEGO spectrophotometer. They will use it to analyze the amount of cranberry juice contained in a juice mix.
Grade Levels: 9-12

What is surface area? How do chemicals bond? What affects properties of materials on the macro- and nano-scale, and why this is important to scientists? Students will do some hands-on activities to find these out.
Grade Levels: 6-12

How do powders react when you add a chemical indicator to them? Students will use their observation skills to find out how different powders react with vinegar, baking soda solution, iodine, and universal pH indicator. Using their observations, they will then need to try to figure out what two powders are mixed together.
Grade Levels: 3-6

What is Vitamin C? Where do you find it and why do we need it? Students will learn about the process of Titration and how chemical reactions can be used to measure or indicate
concentration.Grade Levels: 9-12

What is rust? Students will explore what factors cause metals to rust by experimenting with different metals and solutions. They will then learn about this chemical reaction by looking at redox reactions.
Grade Levels: 6-12